Imagine you have a glass of lemonade. You mix it with a spoon and leave it on the table. When you come back five minutes later, you find that the liquid is still spinning! What’s even weirder, when you pick up the glass, the liquid starts leaking through the bottom – straight through the glass! Not only that, but the liquid is also climbing up the walls of the glass and is flowing out of the top! What is happening here?
Well, if your lemonade was actually a substance called ‘superfluid’, then this is exactly what would happen. But what are superfluids, and why do they have these super freaky characteristics?
Think of when you pour honey or syrup; these liquids have high viscosity and flow quite slowly. A superfluid is a liquid with zero viscosity! That is, it has zero resistance to flow due to the fact that it flows without experiencing frictional forces between its layers.
From what we know, the only element that forms a superfluid when cooled to extremely cold temperatures is helium, which we usually see in it’s gas state. However, only specific isotopes (an atom of an element that has the same number of protons but different numbers of electrons) of helium, helium-4 and helium-3, can be cooled to form a superfluid. This is due to the fact that these isotopes behave like a type of particle known as a ‘boson’. These particles are known for their ability to occupy the same energy level as each other – we will discuss more on that soon. Helium has an interesting characteristic that separates it from the rest – unlike all other elements, helium never freezes to become a solid, even at absolute zero (0 Kelvin or -273 °C), which is the lowest temperature possible. Instead, helium-4 becomes a superfluid at around -270 °C or 2 Kelvin. You may be asking; why does this happen?
As helium-4 cools down, the atoms begin to occupy lower and lower energy states. Eventually, more and more of the atoms occupy the same low energy, or quantum, state – this is when the interesting part takes place. According to quantum mechanics, when atoms are on the same energy level, they begin to behave in unison. The atoms don’t bump into each other or even move in different directions like they would in a regular liquid – they all move together! This is why a superfluid experiences zero internal friction – and why, when you mix a superfluid, it will never stop spinning. Furthermore, superfluids have the ability to slip through anything; itself, and even the walls of their container!
Source: https://www.youtube.com/watch?v=ntyh9VJvq2w&t=359s
In the beginning, we mentioned that superfluids can even climb up the walls of its container and flow out the top – how does this happen? The thing is, all liquids have the tendency to climb the walls of the container it is in, due to the forces of adhesion between the liquid and the walls of the container and the forces of cohesion between the molecules of the liquid – this is why a meniscus forms when you pour a liquid in a test tube! However, the liquid doesn’t go far due to the fact that the frictional forces between the walls of the container and the liquid are usually able to counter that effect. On the other hand, as we know, a superfluid does not experience any friction between its molecules – which is why it is able to travel up the walls of its container, as if out of a sci-fi movie!
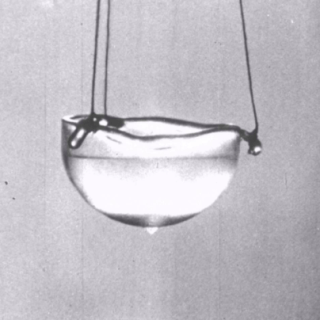
By Manaal Ahmed
Source: https://www.popularmechanics.com/science/a28608/superfluids-absolute-zero-spacetime/
This extremely fascinating substance has uses in precision measurement devices, for example in extremely sensitive gyroscopes used in navigation systems. It can also be used to detect microscopic leaks in containers and vacuum systems. Superfluids may also be found in outer space – scientists think that superfluids may make up the core of neutron stars, which are tiny, highly dense celestial objects in space made up almost entirely of neutrons. There is even the theory that spacetime itself may be a superfluid!
To conclude, superfluids represent how much we really still have to learn about this world, and what strange, amazing things are waiting to be discovered!
Bibliography:
- https://www.youtube.com/watch?v=zJblFBwqjPo&t=334s
- https://www.youtube.com/watch?v=ntyh9VJvq2w&t=359s
- https://www.popularmechanics.com/science/a28608/superfluids-absolute-zero-spacetime/
- https://www.britannica.com/science/superfluidity
- https://www.sciencedirect.com/topics/materials-science/superfluid-helium#:~:text=Under%20standard%20pressure%20and%20temperature%20helium%20is%20a%20gas.,pressure%20(Wilks%2C%201967).
- https://www.usgs.gov/special-topics/water-science-school/science/water-meniscus#:~:text=Water%20molecules%20are%20attracted%20to,the%20glass%2C%20forming%20the%20meniscus.


Leave a comment